Business
A Brave New World

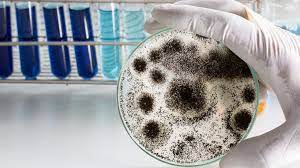
Flourish Labs is testing legal psilocybin in Oregon.
When Oregon voters passed Proposition 109 in 2020, they cleared a path for greater access to the therapeutic use of psilocybin mushrooms and products that contain their active compounds. The ballot measure, which was approved with more than 55% of the vote, authorized the Oregon Health Authority (OHA) to create a program to permit licensed service providers to produce and administer psilocybin-producing mushroom products to adults 21 years of age or older.
A model for progressive drug policy reform, Prop. 109 also laid the groundwork for a new industry in Oregon. The OHA’s Psilocybin Services Section is charged with drafting rules to license and regulate the manufacturing, transportation, delivery, sale, and purchase of psilocybin products as well as the provision of psilocybin services, with a mandate to have the program up and running in 2023. The agency is already accepting applications for psilocybin business licenses and savvy entrepreneurs are launching new enterprises to service a rising industry.
A New Business is Born
George Sellhorn, founder and principal scientist at Flourish Labs in Portland, is one of the business owners preparing for the launch of legal psilocybin in Oregon. He has had a personal relationship with psychedelics, including psilocybin mushrooms, since he was a teenager and acknowledges that psychedelics have had a “huge impact” on his life. He is also an avid cannabis enthusiast and, with tips and encouragement from High Times, has been growing his own plants since 1993. His interest in and passion for cannabis inspired his academic pursuits, with Sellhorn earning a Ph.D. in plant biochemistry from the University of Washington in 2006.
At that time, the legal cannabis industry in the U.S. was in its infancy, and positions in professional fields were few and far between. Sellhorn turned to biotechnology to begin his career, with stints working on cancer therapeutics and an HIV vaccine. Before long, however, friends with businesses in the emerging industry encouraged him to open a cannabis testing lab. Intent on seeing where his chosen path would take, he decided against going into business for himself, although he did dabble in the industry a bit and helped a couple of friends get labs set up. It seemed right for Sellhorn at the time, but it didn’t take long for him to wish he had decided differently.
“A few years later, I kind of was kicking myself saying, ‘I probably should have started a lab, and I’d probably be a lot happier than I am right now,’” he tells me in a telephone interview.
After the passage of Prop. 109, things came full circle. Once again, friends in a soon-to-be legal industry encouraged him to open a lab. The ballot measure includes provisions directing the OHA’s regulations for testing psilocybin products for contamination. Additionally, therapists would want to know the dosage of active compounds they were administering, leading to a need for potency data throughout the supply chain.
Sellhorn remembers thinking, “I’ve been down this road before,” and decided he wouldn’t leave himself open to later regrets this time around. He began ordering the lab equipment and supplies he would need to launch the operation in September 2021, and by the beginning of 2022, Flourish Labs was ready to start taking in samples and running tests.
Sellhorn says that testing mushrooms is quite similar to lab analysis of cannabis, but with one key difference. Like many cannabis labs, Sellhorn uses high-performance liquid chromatography incorporated with ultra-violet spectroscopy (HPLC-UV) to separate the molecules of a given sample and determine its makeup. However, unlike cannabinoids, which are fat-soluble (hydrophobic), the alkaloids in mushrooms are water-soluble (hydrophilic), necessitating a change in the approach to make it work. “So, same methods as cannabis, but just the opposite chemistry,” Sellhorn summarizes.
Lab Testing for Psilocybin, and More
Much of the time Sellhorn spends in testing involves determining the amount of psychoactive alkaloids, or potency, a particular sample contains. More than 50 species of mushrooms produce psilocybin, which is expressed at different levels determined by factors including genetics and cultivation practices.
“The most potent mushroom that I’ve seen from different people is an Albino Penis Envy or an APE,” says Sellhorn. “Eve tested anywhere from 0.1% alkaloids, up to 2.3% was the highest one that I’ve tested so far. So, there’s a pretty big range. The average, I’d say, is about 0.5% to 0.7% alkaloids [by dry weight].”
Initially, Sellhorn’s business plan primarily involved analyzing mushrooms that contain psilocybin and related alkaloids, including psilocin, psilocybin, norpsilocin, baeocystin, and norbaeocystin. Since opening Flourish Labs, he has also developed testing protocols for other products made with psilocybin mushrooms that are likely to be part of Oregon’s upcoming regulated market.
“I can also do fruiting bodies and gummies, chocolates, and extracts, whether it be liquid extract or dry extract” he explains. “So, I have a protocol for all of the possible products that could be made, that I’m aware of, as of now.”
Dosage is Key
Sellhorn notes that the renewed interest in the reported health and wellness benefits of psilocybin has fostered a new culture of microdosing, which Sellhorn has been practicing for more than four years. To microdose, only a tiny fraction of a psychedelic dose of psilocybin is taken, perhaps 0.1 to 0.2 milligrams, Sellhorn suggests. With mushrooms of average potency (rounded up to 1% total alkaloids), that translates to about a tenth to two-tenths of a gram of mushroom biomass. “That’s like a really nice microdose, and you can adjust it based on body weight,” he says. “A microdose should be enough to lift your mood but not feel any of the psychedelic effects like you’re about to trip.”
At the other end of the spectrum is macrodosing, which involves taking enough psilocybin to produce a strong psychedelic effect, which can either be a heck of a fun trip or a space for life-changing spiritual or psychological breakthroughs, depending on the intention with which the drug is taken. To macrodose, Sellhorn says a dosage of 30 milligrams to 50 milligrams of psilocybin (approximately 5 grams of mushroom biomass) should be about right for an intense trip. And within the extremes of micro and macrodosing, “there’s doses in between there for whatever you’re looking for.”
In addition to potency, Sellhorn notes that the form of psilocybin taken can also influence the effects of the drug. While eating dried mushrooms is the classic method of consumption, extracted psilocybin and products made from it can modify the drug’s effects.
“It’s abundantly clear to me now that the mushroom biomass itself acts like a time-release capsule. So, if you take a mushroom that has, say, five milligrams of psilocybin in it, and you eat that, you’ll get a certain effect,” he explains. “And it’ll take a certain amount of time to hit you. But if you take five milligrams in a gummy or a chocolate, it hits you way faster, it’s much more intense, and it gets over more quickly.”
Sellhorn’s work in the lab has given him an opportunity to increase his knowledge about other psilocybin best practices, as well. He notes that proper storage is very effective at preserving the potency of psilocybin mushrooms. When a client was looking for data on potency degradation, an in-house study determined that mushrooms stored in a vacuum-sealed bag and kept in dark conditions at 60° Fahrenheit retained 98% of their potency after four months.
An Expanding Scientific Field
Although he sees a strong market for analyzing psilocybin-containing mushrooms coming to Oregon, Sellhorn realized that demand for lab testing may be limited until the industry is more established and generating revenue. Although the state’s regulations will likely eventually include requirements for testing for microbial contamination or the presence of heavy metals in addition to potency, such testing is not yet in high demand. So, to supplement his business plan, Flourish Labs has also begun lab testing of so-called functional mushrooms including cordyceps, reishi, and amanita muscaria (famous in folklore and pop culture) for compounds that could have health and wellness benefits. Additional species to be tested by the lab in the coming months include lion’s mane, chaga, maitake, tremella, and turkey tail.
When regulated production and administration of psilocybin for therapeutic purposes begins in Oregon later this year, it will launch a new industry in the state and become a milestone in the continued evolution of drug policy reform. Leading the way will be a new generation of innovators and entrepreneurs, including Sellhorn and Flourish Labs.
Source: https://hightimes.com/news/oregon/a-brave-new-world/
Business
New Mexico cannabis operator fined, loses license for alleged BioTrack fraud

New Mexico regulators fined a cannabis operator nearly $300,000 and revoked its license after the company allegedly created fake reports in the state’s traceability software.
The New Mexico Cannabis Control Division (CCD) accused marijuana manufacturer and retailer Golden Roots of 11 violations, according to Albuquerque Business First.
Golden Roots operates the The Cannabis Revolution Dispensary.
The majority of the violations are related to the Albuquerque company’s improper use of BioTrack, which has been New Mexico’s track-and-trace vendor since 2015.
The CCD alleges Golden Roots reported marijuana production only two months after it had received its vertically integrated license, according to Albuquerque Business First.
Because cannabis takes longer than two months to be cultivated, the CCD was suspicious of the report.
After inspecting the company’s premises, the CCD alleged Golden Roots reported cultivation, transportation and sales in BioTrack but wasn’t able to provide officers who inspected the site evidence that the operator was cultivating cannabis.
In April, the CCD revoked Golden Roots’ license and issued a $10,000 fine, according to the news outlet.
The company requested a hearing, which the regulator scheduled for Sept. 1.
At the hearing, the CCD testified that the company’s dried-cannabis weights in BioTrack were suspicious because they didn’t seem to accurately reflect how much weight marijuana loses as it dries.
Company employees also poorly accounted for why they were making adjustments in the system of up to 24 pounds of cannabis, making comments such as “bad” or “mistake” in the software, Albuquerque Business First reported.
Golden Roots was fined $298,972.05 – the amount regulators allege the company made selling products that weren’t properly accounted for in BioTrack.
The CCD has been cracking down on cannabis operators accused of selling products procured from out-of-state or not grown legally:
- Regulators alleged in August that Albuquerque dispensary Sawmill Sweet Leaf sold out-of-state products and didn’t have a license for extraction.
- Paradise Exotics Distro lost its license in July after regulators alleged the company sold products made in California.
Golden Roots was the first alleged rulebreaker in New Mexico to be asked to pay a large fine.
Source: https://mjbizdaily.com/new-mexico-cannabis-operator-fined-loses-license-for-alleged-biotrack-fraud/
Business
Marijuana companies suing US attorney general in federal prohibition challenge

Four marijuana companies, including a multistate operator, have filed a lawsuit against U.S. Attorney General Merrick Garland in which they allege the federal MJ prohibition under the Controlled Substances Act is no longer constitutional.
According to the complaint, filed Thursday in U.S. District Court in Massachusetts, retailer Canna Provisions, Treevit delivery service CEO Gyasi Sellers, cultivator Wiseacre Farm and MSO Verano Holdings Corp. are all harmed by “the federal government’s unconstitutional ban on cultivating, manufacturing, distributing, or possessing intrastate marijuana.”
Verano is headquartered in Chicago but has operations in Massachusetts; the other three operators are based in Massachusetts.
The lawsuit seeks a ruling that the “Controlled Substances Act is unconstitutional as applied to the intrastate cultivation, manufacture, possession, and distribution of marijuana pursuant to state law.”
The companies want the case to go before the U.S. Supreme Court.
They hired prominent law firm Boies Schiller Flexner to represent them.
The New York-based firm’s principal is David Boies, whose former clients include Microsoft, former presidential candidate Al Gore and Elizabeth Holmes’ disgraced startup Theranos.
Similar challenges to the federal Controlled Substances Act (CSA) have failed.
One such challenge led to a landmark Supreme Court decision in 2005.
In Gonzalez vs. Raich, the highest court in the United States ruled in a 6-3 decision that the commerce clause of the U.S. Constitution gave Congress the power to outlaw marijuana federally, even though state laws allow the cultivation and sale of cannabis.
In the 18 years since that ruling, 23 states and the District of Columbia have legalized adult-use marijuana and the federal government has allowed a multibillion-dollar cannabis industry to thrive.
Since both Congress and the U.S. Department of Justice, currently headed by Garland, have declined to intervene in state-licensed marijuana markets, the key facts that led to the Supreme Court’s 2005 ruling “no longer apply,” Boies said in a statement Thursday.
“The Supreme Court has since made clear that the federal government lacks the authority to regulate purely intrastate commerce,” Boies said.
“Moreover, the facts on which those precedents are based are no longer true.”
Verano President Darren Weiss said in a statement the company is “prepared to bring this case all the way to the Supreme Court in order to align federal law with how Congress has acted for years.”
While the Biden administration’s push to reschedule marijuana would help solve marijuana operators’ federal tax woes, neither rescheduling nor modest Congressional reforms such as the SAFER Banking Act “solve the fundamental issue,” Weiss added.
“The application of the CSA to lawful state-run cannabis business is an unconstitutional overreach on state sovereignty that has led to decades of harm, failed businesses, lost jobs, and unsafe working conditions.”
Business
Alabama to make another attempt Dec. 1 to award medical cannabis licenses

Alabama regulators are targeting Dec. 1 to award the first batch of medical cannabis business licenses after the agency’s first two attempts were scrapped because of scoring errors and litigation.
The first licenses will be awarded to individual cultivators, delivery providers, processors, dispensaries and state testing labs, according to the Alabama Medical Cannabis Commission (AMCC).
Then, on Dec. 12, the AMCC will award licenses for vertically integrated operations, a designation set primarily for multistate operators.
Licenses are expected to be handed out 28 days after they have been awarded, so MMJ production could begin in early January, according to the Alabama Daily News.
That means MMJ products could be available for patients around early March, an AMCC spokesperson told the media outlet.
Regulators initially awarded 21 business licenses in June, only to void them after applicants alleged inconsistencies with how the applications were scored.
Then, in August, the state awarded 24 different licenses – 19 went to June recipients – only to reverse themselves again and scratch those licenses after spurned applicants filed lawsuits.
A state judge dismissed a lawsuit filed by Chicago-based MSO Verano Holdings Corp., but another lawsuit is pending.
Source: https://mjbizdaily.com/alabama-plans-to-award-medical-cannabis-licenses-dec-1/
-

 Business2 years ago
Business2 years agoPot Odor Does Not Justify Probable Cause for Vehicle Searches, Minnesota Court Affirms
-

 Business2 years ago
Business2 years agoNew Mexico cannabis operator fined, loses license for alleged BioTrack fraud
-

 Business2 years ago
Business2 years agoAlabama to make another attempt Dec. 1 to award medical cannabis licenses
-

 Business2 years ago
Business2 years agoWashington State Pays Out $9.4 Million in Refunds Relating to Drug Convictions
-

 Business2 years ago
Business2 years agoMarijuana companies suing US attorney general in federal prohibition challenge
-

 Business2 years ago
Business2 years agoLegal Marijuana Handed A Nothing Burger From NY State
-

 Business2 years ago
Business2 years agoCan Cannabis Help Seasonal Depression
-

 Blogs2 years ago
Blogs2 years agoCannabis Art Is Flourishing On Etsy